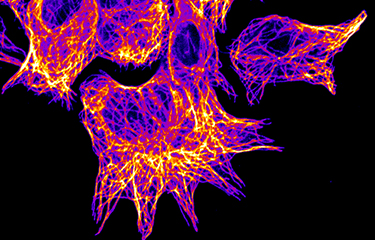
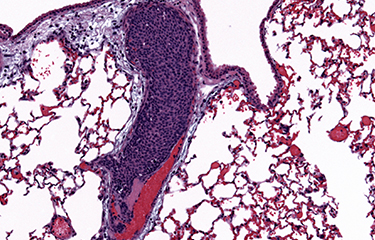
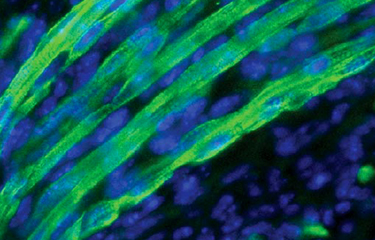
Produire de nouveaux
savoirs
Explorer de nouvelles avenues pour développer la connaissance médicale de demain dans une approche qui intègre recherche fondamentale et recherche clinique.
Nos groupes de recherche sont dirigés par des chercheuses et chercheurs principaux qui collaborent dans un esprit de collégialité et de partage pour rapprocher la recherche des patients et patientes et former la relève scientifique. Ce sont des esprits libres et indépendants qui travaillent sans relâche pour améliorer la santé. Découvrez les chercheurs et chercheuses de l'IRCM.
Recherche en cours
"Nos travaux visent à comprendre les mécanismes moléculaires permettant aux cellules cancéreuses de former des métastases"
Jean-François Côté, directeur de recherche
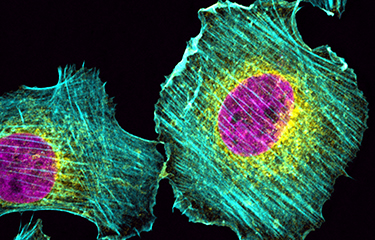
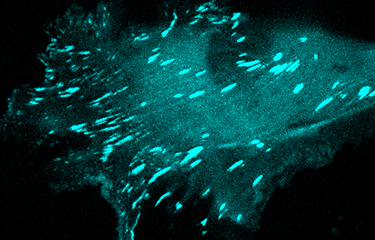
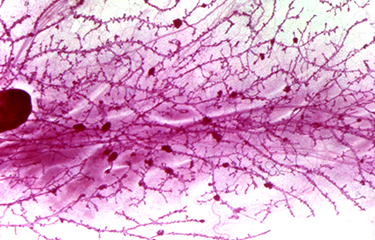
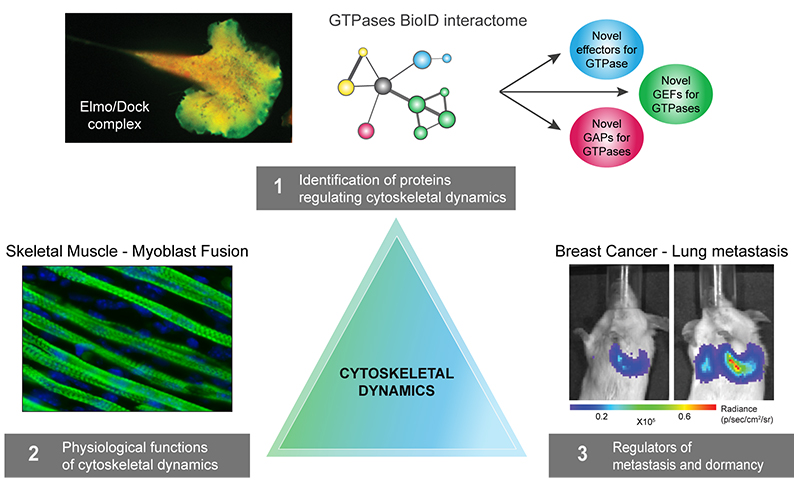
Organisation du cytosquelette et migration cellulaire
Les tumeurs métastatiques sont responsables de 90 % des décès causés par le cancer. La formation de métastases est un processus complexe qui implique la migration et l'invasion de cellules, leurs entrées dans la circulation et se complète quand les cellules cancéreuses envahissent des tissus éloignés avant de se développer en lésions macro-métastatiques. Une fois que la maladie s'étend aux organes secondaires, elle devient pratiquement incurable. La compréhension des mécanismes cellulaires et moléculaires permettant aux cellules cancéreuses de coloniser des organes secondaires et de croître en macro-métastases est essentielle afin de découvrir de nouvelles cibles thérapeutiques qui pourront améliorer la survie des patients. Pour réaliser cet objectif général à long terme, nous avons conçu un programme de recherche original qui utilise des approches pluridisciplinaires visant à comprendre la dynamique du cytosquelette.
| Équipe |
|
| Affiliations |
|
| Formations et expérience |
|
| Publications |
Publications - Chapitres de livres1) Tremblay ML, Charest A, Côté JF, Elchebly M, Wagner J. Towards protein tyrosine phosphatases substrate identification: Combination of biochemical and genetic approaches. In: Yakura H, éd. Kinases and phosphatases in lymphocyte and neuronal signalling. 1997; 23-362) Côté JF, Vuori K. In vitro Guanine Nucleotide Exchange Activity of DHR-2/DOCKER/CZH2 Domains. In: Hall A, Der CJ, éds. Methods in Enzymology, 2006; 406:41-57 3) Côté JF, Vuori K. Discovery of DOCK180 superfamily of exchange factors. In: Pasqualini R, Arap W, éds. Protein Discovery Technologies, 2009; 171-189. (NCIC, 019104) Publications de recherche ou d’érudition dans des revues dotées de comités de pairs (soumises ou en préparation finale)1) El Khoury M, Lalonde A, Robert A, Hipfner D, Côté JF. (2021) The scaffold proteins Tanc1 and Tanc2 are required for myoblast fusion. (Soumis; Scientific Reports)2) Helness A, Fraszczak J, Joly-Beauparlant C, Bagci H, Trahan C, Arman K, Shooshtarizadeh P, Chen R, Ayoub M, Côté JF, Oeffinger M, Droit A, Möröy T. (2021). “GFI1 tethers the NuRD complex to open and transcriptionally active chromatin in myeloid progenitors”. BioRxiv preprint. DOI: 10.1101/2021.05.31.446398. (Soumis; Communications Biology) 3) Leguay K, Decelle B, Elkholi I, Bouvier M, Côté JF, Carréno S. (2021). “Interphase microtubule disassembly is a signaling cue that drives cell rounding at mitotic entry”. (Soumis; Nature). 4) Mehra C, Chung JH, He Y, Lara Márquez M, Goyette MA, Boufaied N, Barrès V, Ouellet V, Guérard KP, Delliaux C, Saad F, Lapointe J, Côté JF, Labbé, D., Lamarche-Vane, N. (2020). “The Rac1/Cdc42 regulator CdGAP promotes prostate cancer metastasis by regulating epithelial-to-mesenchymal transition, cell cycle progression, and apoptosis” (Révisé positivement; Communications Biology). 5) Heath, J., Cheyou, E., Luo V.M., Samiei A., Lee J., Chen X., Morin T., Djerir B., Karam M., Bagci, H., Grapton D., Ursini-Siegel J., Côté J.F., Richard R., Maréchal A., Orthwein A. (2021) “POGZ modulates the DNA damage response in a HP1-dependent manner”. (Soumis; EMBO Reports). 6) Tran, V., Desanlis, I., Killoran, R., Ravichandran, K.S., Smith, M.J., Kmita, M., Côté, J.F. (2021) “Manipulation of the myoblast fusion process can be exploited for regenerative therapies in muscle diseases”. (Soumis, Nature; MS-2021-01-01066). 7) Tran V., Nahlé S., Côté, J.F. (2021) “Curtain raised for a myoblast fusion play: the actors that shape cytoskeletal dynamics for membranes coalescence”. (Demande de pré-soumission recue favorablement; Current Biology) Publications de recherche ou d’érudition dans des revues dotées de comités de pairs (publiées)1) Côté JF, Roberge AG* (1996). Nitric oxide synthase in cat brain: Cofactors-enzyme-substrate interaction. Free Radic Biol Med 1996; 21:109-115. 2) Côté JF, Charest A, Wagner J, Tremblay ML* (1998). Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry 1998; 37:13128-13137. 3) Angers-Loustau A, Côté JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML* (1999). Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol 1999; 144:1019-1031. 4) Nishiya N, Iwabuchi Y, Shibanuma M, Côté JF, Tremblay ML, Nose K* (1999). Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM3 domain. J Biol Chem 1999; 274:9847-9853. 5) Côté JF, Turner CE, Tremblay ML* (1999). Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J Biol Chem 1999; 274:20550-20560. 6) Angers-Loustau A, Côté JF , Tremblay ML* (1999). Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochem Cell Biol 1999; 77:493-505. 7) Cong F, Spencer S, Côté JF, Wu Y, Tremblay ML, Lasky LA, Goff SP* (2000). Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol Cell 2000; 6:1413-1423. 8) Côté JF, Chung PL, Théberge JF, Hallé M, Spencer SD, Lasky LA, Tremblay ML * (2002). PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem 2002; 277:2973-2986. 9) Côté JF, Vuori K* (2002). Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci 2002; 115:4901-4913. 10) Liu E, Côté JF, Vuori K* (2003). Negative regulation of FAK signalling by SOCS proteins. EMBO J. 2003; 22:5036-5046. 11) Dail M, Kalo MS, Seddon JA, Côté JF, Vuori K, Pasquale EB* (2004). SHEP1 function in cell migration is impaired by a single amino acid mutation that disrupts association with the scaffolding protein Cas but not with Ras GTPases. J Biol Chem 2004; 279:41892-41902. 12) Côté JF, Motoyama AB, Bush JA, Vuori K* (2005). A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 2005; 7:797-807. 13) Côté JF, Vuori K* (2006). In vitro guanine nucleotide exchange activity of DHR-2/DOCKER/CZH2 domains. Methods Enzymol 2006; 406, 41-57. 14) Sastry SK*, Rajfur Z, Liu BP, Côté JF, Tremblay ML, Burridge K (2006). PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J Biol Chem 2006; 281:11627-11636. (CIHR, MOP-77591) 15) Sirois J, Côté JF, Charest A, Uetani N, Bourdeau A, Duncan SA, Daniels E*, Tremblay ML* (2006). Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech Dev 2006; 123:869-880. (CIHR, MOP-77591) 16) Côté JF*, Vuori K* (2007). GEF What? DOCK180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007; 17:383-393. (CIHR, MOP-77591) (*Co-auteurs seniors) 17) Komander D*, Patel M*, Laurin M, Fradet N, Pelletier A, Barford D, Côté JF (2008). An Alpha-Helical Extension of the ELMO1 Pleckstrin Homology Domain Mediates Direct Interaction to DOCK180 and Is Critical in Rac Signalling. Mol Biol Cell 2008; 19:4837-4851. (CIHR, MOP-77591) (*Co-premiers auteurs) 18) Laurin, M., Fradet, N., Blangy, A., Hall, A., Vuori, K.*, Côté, J.F.*. (2008). The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci USA, 105:15446-15451. (CIHR, MOP-77591) (*Co-auteur senior) 19) Côté JF*, Vuori K* (2009) Cell Biology. Two Lipids That Give Direction. Science 2009; 324:346-347. (CIHR, MOP-77591) (*Co-auteur senior) 20) Premkumar L, Bobkov AA, Patel M, Jaroszewski L, Bankston LA, Stec B, Vuori K, Côté JF, Liddington RC* (2010). Structural basis of membrane-targeting by the Dock180 family of Rho-family Guanine Exchange Factors (RhoGEFs). J Biol Chem 2010; 285:13211-13222. (CIHR, MOP-77591) 21) Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, Ding G, Noda M, Murata Y, Tanaka Y, Masuko S, Suda T, Meno C, Côté JF, Nagasawa T, Fukui Y* (2010). DOCK180 Is a Rac Activator That Regulates Cardiovascular Development by Acting Downstream of CXCR4. Circulation Research 2010; 107:1102-1105. (CIHR, MOP-77591) 22) El-Bikai R, Welman M, Margaron Y, Côté JF, Macqueen L, Buschmann MD, Fahmi H, Shi Q, Maghni K, Fernandes JC, Benderdour M* (2010). Perturbation of Adhesion Molecule-mediated Chondrocyte-Matrix Interactions by 4-Hydroxynonenal Binding: Implication in Osteoarthritis Pathogenesis. Arthritis Research & Therapy 2010; 12:R201. (NCIC, 019104) 23) Patel M*, Margaron Y*, Fradet N, Yang Q, Wilkes B, Bouvier M, Hofmann K, Côté JF (2010). An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signalling. Current Biology 2010; 20:2021-2027. (CIHR, MOP-77591) (*Co-premiers auteurs) 24) Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A* (2011). The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. Journal of Bone and Mineral Research 2011; 26:1099-1110. (CIHR, MOP-77591) 25) Patel M, Chiang TC, Tran V, Lee F-JS, Côté JF (2011). The Arf family GTPase Arl4A complexes with ELMO proteins to promote actin cytoskeleton remodeling and reveals a versatile Ras-binding domain in the ELMO proteins family. J Biol Chem 2011; 286:38969-38979. (CIHR, MOP-77591) 26) Patel M, Pelletier A, Côté JF* (2011). EXTRA VIEWS: Opening up on ELMO regulation: New insights into the control of Rac signalling by the DOCK180/ELMO complex. Small GTPases 2011; 2:268-275. (CIHR, MOP-77591) 27) Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, Zon LI, Côté JF, Gratton JP* (2012). Formation of a PKCζ/β-catenin complex in endothelial cells promotes angiopoietin-1- induced collective directional migration and angiogenic sprouting. Blood 2012; 120:3371-3381. (CCSRI-019104) 28) Margaron Y, Fradet N, Côté JF* (2013). ELMO recruits Actin Cross-linking Family 7 (ACF7) at the cell membrane for microtubule capture and stabilization of cellular protrusions. J Biol Chem 2013; 288:1184-1199. (CCSRI-019104/CIHR, MOP-77591) 29) Wong WP, Altemus JB, Hester JF, Chan ER, Côté JF, Serre D, Sehayek E* (2013). Cathepsin B is a novel gender-dependent determinant of cholesterol absorption from the intestine. J Lipid Res 2013; 54:816-822. 30) Sanematsu F, Nishikimi A, Watanabe M, Hongu T, Tanaka Y, Kanaho Y, Côté JF, Fukui Y* (2013). Phosphatidic acid-dependent recruitment and function of the Rac activator DOCK1 during dorsal ruffle formation. J Biol Chem 2013;288:8092-8100. (CCSRI-019104) 31) Laurin M, Huber J, Pelletier A, Houalla T, Park M, Fukui Y, Haibe-Kains B, Muller WJ, Côté JF* (2013). Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci USA 2013;110:7434-7439. (CCSRI-019104). Cette publication a reçu le Prix pour les meilleures publications de l’Institut du cancer (IC) 2013-2014, et parmi Les percées les plus remarquables des chercheurs de la Société canadienne du cancer en 2013 32) Patel M, Côté JF* (2013). Ras GTPases' interaction with effector domains: Breaking the families' barrier. Communicative and Integrative Biology 2013; 6:e24298. 33) Laurin M, Dumouchel A, Fukui Y, Côté JF* (2013). The Rac-specific exchange factors Dock1 and Dock5 are dispensable for the establishment of the glomerular filtration barrier in vivo. Small GTPases 2013; 4:221-230. (CCSRI-019104) 34) Hamoud N, Tran V, Croteau LP, Kania A, Côté JF* (2014). G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc Natl Acad Sci USA 2014;111:3745-3750. (CIHR, MOP-77591) 35) Laurin M, Côté JF* (2014). Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes and Development 2014;28:533-547. (CCSRI-019104/CIHR, MOP-77591/CRS/QBCF) 36) Ogawa K, Tanaka Y, Uruno T, Duan X, Harada Y, Sanematsu F, Yamamura K, Terasawa M, Nishikimi A, Côté JF, Fukui Y* (2014). DOCK5 functions as a key signalling adaptor that links FcεRI signals to microtubule dynamics during mast cell degranulation. The Journal of Experimental Medicine 2014; 211:1407-1419. 37) Bagci H, Laurin M, Huber J, Muller WJ et Côté JF* (2014). Impaired cell death and mammary gland involution in the absence of Dock1 and Rac1 signalling. Cell Death and Diseases 2014;5:e1375. (CCSRI, 019104/CIHR, MOP-77591) 38) Goyette MA, Côté JF* (2014). NSCLC metastasis: Going with ELMO3. Oncotarget 2014; 5: 5850-5851. 39) Watanabe M, Terasawa M, Miyano K, Yanagihara T, Uruno T, Sanematsu F, Nishikimi A, Côté JF, Sumimoto H, Fukui Y* (2014). DOCK2 and DOCK5 Act Additively in Neutrophils to Regulate Chemotaxis, Superoxide Production and Extracellular Trap Formation. The Journal of Immunology 2014; 193:5660-5667. (CIHR, MOP-77591) 40) Abu-Thuraia A, Gauthier R, Chidiac R, Fukui Y, Screaton RA, Gratton JP, Côté JF* (2015). Axl phosphorylates Elmo scaffold proteins to promote Rac activation and cell invasion. Molecular and Cellular Biology 2015; 35:76-87. (QBCF) 41) Sun Y, Ren W, Côté JF, Hinds PW, Hu X, Du K* (2015). ClipR-59 Interacts with Elmo2 and modulates myoblast fusion. J Biol Chem 2015; 290:6130-6140. (CIHR, MOP-77591) 42) Sun Y, Côté JF, Du K* (2016). Elmo2 Is a Regulator of Insulin-dependent Glut4 Membrane Translocation. J Biol Chem 2016; 291(31): 16150-61. 43) Dusseault J, Li B, Haider N, Goyette MA, Côté JF, Larose L* (2016). Nck2 deficiency in mice results in increased adiposity associated with adipocyte hypertrophy and enhanced adipogenesis. Diabetes 2016; 65:2652-2666. (CIHR, MOP-77591) 44) He Y, Northey JJ, Pelletier A, Kos Z, Meunier L, Haibe-Kains B, Mes-Masson AM, Côté JF, Siegel PM, Lamarche-Vane N* (2017). The Cdc42/Rac1 regulator CdGAP is a critical E-cadherin transcriptional co-repressor with Zeb2 in breast cancer. Oncogene 2017;36:3490-3503. (CIHR, MOP 142425) 45) Tajiri H, Uruno T, Shirai T, Takaya D, Matsunaga S, Setoyama D, Watanabe M, Kukimoto-Niino M, Oisaki K, Ushijima M, Sanematsu F, Honma T, Terada T, Oki E, Shirasawa S, Maehara Y, Kang D, Côté JF, Yokoyama S, Kanai M, Fukui Y* (2017). Targeting Ras-Driven Cancer Cell Survival and Invasion through Selective Inhibition of DOCK1. Cell Reports 2017;19, 969-980. 46) Robins R, Baldwin C., Aoudjit L., Côté J.F., Gupt I.R., Takano T* (2017). Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney International 2017; 92,349-364. 47) Hernandez-Vasquez MN, Adame-Garcia S, Hamoud N, Chidiac R, Reyes-Cruz G, Gratton JP, Côté JF, Vazquez-Prado, J* (2017). Cell adhesion controlled by adhesion G protein-coupled receptor GPR124/ADGRA2 is mediated by a protein complex comprising intersectins and Elmo-Dock. J Biol Chem 2017,292, 12178-12191. 48) Duhamel S, Goyette MA, Thibault MP, Filion D, Gaboury L, Côté JF* (2018). The E3 ubiquitin ligase HectD1 suppresses EMT and metastasis by targeting the +TIP protein ACF7 for degradation. Cell Reports 2018;22, 1016-1030. 49) Youn JY, Dunham WH, Hong SJ, Knight JDR, Chen GI, Bagci H, Bashkurov M, Rathod B, MacLeod G, Eng SWM, Angers S, Morris Q, Fabian M, Côté JF, Gingras AC* (2018). High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Molecular Cell 2018;69(3), 517-532.e11. 50) Vadnais C, Chen R, Fraszczak J, Yu Z, Boulais J, Pinder J, Frank D, Khandanpour C, Hébert J, Dellaire G, Côté JF, Richard S, Orthwein A, Drobetsky E, Möröy T* (2018). GFI1 is required for efficient DNA 1 Repair by regulating PRMT1 dependent 2 methylation of MRE11 and 53BP1. Nature Communications 2018;9, 1418. 51) Tomino T, Tajiri H, Tatsuguchi T, Shirai T, Oisaki K, Matsunaga S, Sanematsu F, Sakata D, Yoshizumi T, Maehara Y, Kanai Motomu, Côté JF, Fukui Y, Uruno T* (2018). DOCK1 inhibition suppresses cancer cell invasion and macropinocytosis induced by self-activating Rac1P29S mutation. Biochem. Biophys. Res. Commun 2018;497, 298-304. 52) Methot SP, Litzler LC, Subramani PG, Eranki AK, Fifield H, Patenaude AM, Gilmore JC, Bagci H, Santiago GE, Coté JF, Larijiani M, Verdun RE, Di Noia JM* (2018). A licensing step links AID to transcription elongation for mutagenesis in B cell. Nature Communications, 2018;9, 1248. 53) Goyette MA, Duhamel S, Aubert L, Pelletier A, Savage P, Johnson RM, Carmeliet P, Basik M, Gaboury L, Muller WJ, Park M, Roux PP, Gratton JP, Côté JF* (2018). The Receptor Tyrosine Kinase AXL is Required at Multiple Steps of the Metastatic Cascade during HER2-positive Breast Cancer Progression. Cell Reports 2018;23, 1476-1490. 54) Makihara S, Morin S, Côté JF, Yam PT*, Charron F* (2018). Polarized Dock Activity Drives Shh-Mediated Axon Guidance. Developmental Cell 2018;46(4), 410-425. e7. doi: 10.1016/j.devcel.2018.07.007. 55) Findlay S, Heath J, Luo V, Malina A, Morin T, Djerir B, Li Z, Samiei A, Simo-Cheyou E, Karam M, Bagci H, Rahat D, Grapton D, Lavoie EG, Dove C, Khaled H, Kuasne H, Mann KK, Klein K, Greenwood CM, Tabach Y, Park M, Côté JF, Maréchal A, Orthwein A* (2018). SHLD2/FAM35A co-operates with REV7 to coordinate DNA double-strand break repair pathway choice. EMBO J 2018;37(18). pii: e100158. doi: 10.15252/embj.2018100158. 56) Hamoud N, Tran V, Aimi T, Kakegawa W, Lahaie S, Thibault MP, Pelletier A, Wong GW, Kim IS, Kania A, Yuzaki M, Bouvier M, Côté JF* (2018). Spatiotemporal regulation of G-Protein Coupled Receptor BAI3 canonical and non-canonical signaling by C1q-Like proteins and Stabilin-2 controls myoblast fusion. Nature Communications 2018;9, 4470. doi: 10.1038/s41467-018-06897-5. 57) Lahaie S, Morales D, Bagci H, Hamoud N, Castonguay CE, Kazan JM, Desrochers G, Klar A, Gingras AC, Pause A, Côté JF, Kania A* (2019). The endosomal sorting adaptor HD-PTP is required for ephrin-B:EphB signalling in cellular collapse and spinal motor axon guidance. Sci Rep 2019; 9(1):11945. doi: 10.1038/s41598-019-48421-9. PMID: 31420572 PMCID: PMC6697728. 58) Goyette MA, Cusseddu R, El Kholi I, El-Hachem N, Haibe-Kains B, Gratton JP, Côté JF* (2019). AXL knockdown gene signature reveals a drug repurposing opportunity for a class of antipsychotics to reduce growth and metastasis of triple-negative breast cancer. Oncotarget 2019; 12;10(21):2055-2067. doi: 10.18632/oncotarget.26725. 59) Shooshtarizadeh P, Helness A, Vadnais C, Brouwer N, Beauchemin H, Chen R, Bagci H, Staal FJT, Côté JF, Möröy T* (2019). Gfi1b regulates the level of Wnt/β-catenin signaling in hematopoietic stem cells and megakaryocytes. Nature Communications 2019;10(1):1270. doi: 10.1038/s41467-019-09273-z . 60) Bagci H, Sriskandarajah N, Robert A, Boulais J, Elkholi I E, Tran V, Lin ZY, Thibault MP, Dubé N, Faubert D, Hipfner DR, Gingras AC, Côté JF* (2020). Mapping the proximity interaction network of the RHO-family GTPases reveals novel signaling pathways and regulatory mechanisms. Nature Cell Biology 2020. 22(1):120-134. doi: 10.1038/s41556-019-0438-7. 61) Binet F, Cagnone G, Neault M, Dejda A, Hata M, Wilson A, Buscarlet M, Guber V, Laflamme R, Crespo-Garcia S, Mawambo- Tagne G, Sawchyn C, Abu-Thuraia A, Côté JF, Andelfinger G, Rezende FA, Sennlaub F, Joyal JS*, Mallette F*, Sapieha P* (2020). Neutrophil Extracellular Traps Target Senescent Vasculature For Tissue Remodeling in Retinopathy. Science 2020. 369(6506), eaay5356. doi: 10.1126/science.aay5356. 62) Duval S, Abu-Thuraia A, Elkholi I, Chen R, Seebun D, Mayne J, Côté JF, Figeys D, Seidah N*. (2020) Shedding of cancer susceptibility candidate 4 by the convertases PC7/furin unravels a novel secretory protein implicated in cancer progression. Cell Death Dis 2020. 11, 665. https://doi.org/10.1038/s41419-020-02893-0. 63) Chang L, Yang J, Jo CH, Boland A, Zhang Z, McLaughlin SH, Abu-Thuraia A, Killoran RC, Smith MJ, Côté, JF, Barford D*. (2020). Structure of the DOCK2−ELMO1 complex provides insights into regulation of the auto-inhibited state. Nature Communications (2020). 11: 3464. https://doi.org/10.1038/s41467-020-17271-9. 64) Abu-Thuraia A, Goyette M., Boulais J, Delliaux C, Boulais J, Chidiac R, Schott C, Bagci H, Thibault MP, Davidson D, Ferron M, Veillette A, Daly RJ, Gingras AC, Gratton JP, Côté JF *. (2020). AXL confers cell migration and invasion by hijacking a PEAK1-regulated focal adhesion protein network. Nature Communications 0. 11, 3586. https://doi.org/10.1038/s41467-020-17415-x. 65) Tran V, Martínez M*, Goyette MA*, Jiménez de Domingo A, Tirado P, Fernández-Mayoralas DM, Fernández-Perrone AL, Calleja-Pérez B, Álvarez S, Côté JF*, Fernández-Jaén A* (2020) Biallelic ELMO3 mutations causes an autism spectrum disorder through loss of function for DOCK-mediated RAC1 activation. Small GTPases 2020. 1-8. https://doi.org/10.1080/21541248.2021.1888557. (version préliminaire publiée en ligne; co-auteur senior) 66) Goyette MA, Elkholi I, Apcher C, Kuasne H, Rothlin C, Muller W, Richard D, Park M, Gratton JP, Côté JF*, (2021). Targeting AXL favors an Anti-Tumorigenic Tumor Microenvironment that enhances Immunotherapy Responses by decreasing HIF-1a levels in cancer cells. PNAS 2021. 118(29). https://doi.org/10.1073/pnas.2023868118. 67) Elkholi, I., Di Iorio, M., Fahiminiya, S., Arcand, S.L., Han, H., Nogué, C., Behl, S., Hamel, N., Giroux, S., De Ladurantaye, M., Aleynikova, O., Gotlieb, W.H., Côté, J.F., Rousseau, F., Tonin, P.N., Provencher, D., MesMasson, A.M., Akbari, M., Rivera, B., Foulkes, W. (2021). Investigating the causal role of MRE11A p.E506* in breast and ovarian cancer. Scientific Reports 2021. 11, 2409. https://doi.org/10.1038/s41598-021-81106-w. 68) Cusseddu R, Robert A*, Côté JF* (2021) Strength through unity: the power of the mega-scaffold MACF1. Front Cell Dev Biol 2021. doi: 10.3389/fcell.2021.641727. (accepté; version préliminaire publiée en ligne) 69) Zolotarov, Y., Ma, C., González-Recio, I., Hardy, S., Franken, G., Kostantin, E., Latta, F., Uetani, N., Boulais, J., Thibault, M.P., Côté J.F., Díaz Moreno I., Díaz Quintana A., Hoenderop J.G.J., Martínez-Cruz L.A., Tremblay M.L., de Baaij J.H.L. (2021) "ARL15 modulates magnesium homeostasis through N-glycosylation of CNNMs”. Cellular and Molecular Life Sciences. doi: 10.1101/2020.09.09.289835. (accepté; version préliminaire publiée en ligne) |
Comprendre les bases moléculaires de la migration cellulaire.
Nous exploitons la protéomique moderne (par exemple le BioID), la biologie cellulaire et les approches biochimiques comme des outils pour découvrir de nouveaux gènes impliqués dans la dynamique du cytosquelette et la migration cellulaire en mettant l'accent sur la signalisation par les membres des différentes familles de petites GTPases.
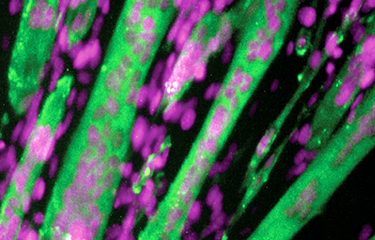
Dynamique du cytosquelette in vivo: déchiffrer les voies de signalisation contrôlant la fusion des myoblastes.
Les cellules cancéreuses exploitent souvent à leur avantage des voies de signalisation importantes pour le développement. En tant que tel, nous visons à déterminer le rôle de nouveaux régulateurs de la dynamique du cytosquelette identifiés dans le Thème-1 dans leurs contextes physiologiques. Nos travaux sur DOCK1, ELMO1/2 et BAI3 nous ont mené à découvrir les premières molécules requises pour la fusion des myoblastes chez les vertébrés.
Vers l'identification de cibles thérapeutiques impliquées dans la formation des métastases.
Notre programme actuel sur le cancer du sein est divisé en deux problématiques majeures. Question 1: nous voulons identifier et caractériser les protéines qui favorisent l’invasion cellulaire et la formation de métastases car elles pourraient être candidates au développement de thérapies anti-métastatiques (par exemple DOCK1, AXL et ACF7). Question 2: les cellules cancéreuses disséminées et dormantes représentent des bombes à retardement pour les patientes. Il est actuellement impossible de prédire quand ces cellules seront réactivées pour former des macro-métastases mortelles. Nous développons actuellement une nouvelle ligne de recherche pour commencer à identifier les gènes participant à la réactivation des cellules dormantes in vivo.
Découvertes importantes
|
2020 Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms |
|
2020 AXL confers cell migration and invasion by hijacking a PEAK1-regulated focal adhesion protein network |
|
2018 The Receptor Tyrosine Kinase AXL Is Required at Multiple Steps of the Metastatic Cascade during HER2-Positive Breast Cancer Progression |
|
2018 Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion |
|
2018 The E3 Ubiquitin Ligase HectD1 Suppresses EMT and Metastasis by Targeting the +TIP ACF7 for Degradation |
|
2013 Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis |
|
2008 The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo |
Outils
| Revue de presse |
|
| Subventions |
2021/09-2023/08 2021/08-2026/07 2021/08-2026/07 2016/04-2022/03 2017/04-2022/03 2020/09-2022/9 |
| Reconnaissances et honneur |
2013/11 2013/12 2014/08 |
Rejoindre
le groupe
de recherche
Postes disponibles
Études cliniques en cours
Partenaires de recherche





Contacter le
groupe de recherche
514 987-5656
jean-francois.cote@ircm.qc.ca

© Institut de recherches cliniques de Montréal, Année.Tous droits réservés. | Politique sur la protection des renseignements personnels | Conditions d'utilisation | Réalisation Agence Riposte